Restore Operational Stability in 90-120 Days
When your pharmaceutical manufacturing operations are off-track, GMPKit deploys our proven approach, expert network, and digital tools to turn strategy into disciplined execution—fast.
When Manufacturing Strategy Breaks Down
GMPKit engages when operational crises threaten your site performance. Which challenge are you facing?
Batch Success
Low first-pass success rates driving up COPQ and delaying product delivery
Backlog Reduction
Growing deviation and investigation backlogs creating regulatory risk
Scale-Up
Struggling to scale from clinical to commercial manufacturing volumes
Inspection Readiness
FDA inspection imminent or findings requiring rapid remediation
Process Optimization
Inefficient business processes and workflow bottlenecks limiting batch throughput
Complex Deviation
Investigation stalled on complex deviation requiring specialized expertise
The GMPKit Approach to Strategy Deployment
We deliver results faster than internal teams by bringing the right approach and toolset to restore operational stability in 90-120 days.
Scope & Discovery
Understand strategic intent and diagnose current state
Strategy Translation & Toolkit Selection
Match the right tools to your specific challenges
Implementation
Partner with your site teams to deploy solutions in 90-120 days
Improvement Monitoring
Ensure sustainable results
Hover over or tap any step to learn more
Our Toolkit: Experience, Tools, and Expert Network
Our experience, tools, and SME network help us solve problems quickly—getting your strategy back on track.
Diagnostic Frameworks
Identify gaps that cause strategy to fail across leadership, compliance, operations, and quality
SME Network
Expert intervention when your team is stuck—deploying specialized expertise to unblock progress
Benchmarking Intelligence
Understand industry standards and turn operational reliability into a competitive advantage
Leadership Development
Build critical skills to deploy strategy in highly regulated GMP environments
"Sprint" Governance Model
LEAN + PDCA + Agile governance framework for weekly planning, execution, and strategy checks
LEAN Frameworks
Structured problem-solving approaches perfected for pharmaceutical manufacturing
BatchTrak™ Platform
Digital visibility and accountability for batch operations—streamlining disposition and driving actions
Consulting + Agency Model
Unlike traditional consultants who provide advice, we partner directly with your site teams and assume accountability for measurable results.
90-120 Day Commitment
We deliver measurable results in 90-120 days, not 6-12 month projects. When your manufacturing network is in crisis, speed matters.
Execution, Not Just Advice
We work alongside your site teams to execute solutions—not deliver recommendations and leave. We assume accountability for results.
Expert Intervention
We deploy our SME network when your team needs specialized expertise or bandwidth—bringing process development, quality, and operations experts.
Proven Across CDMOs
Validated across mid-size CDMOs, biopharma sites, cell therapy operations, and gene therapy facilities—we understand your challenges.
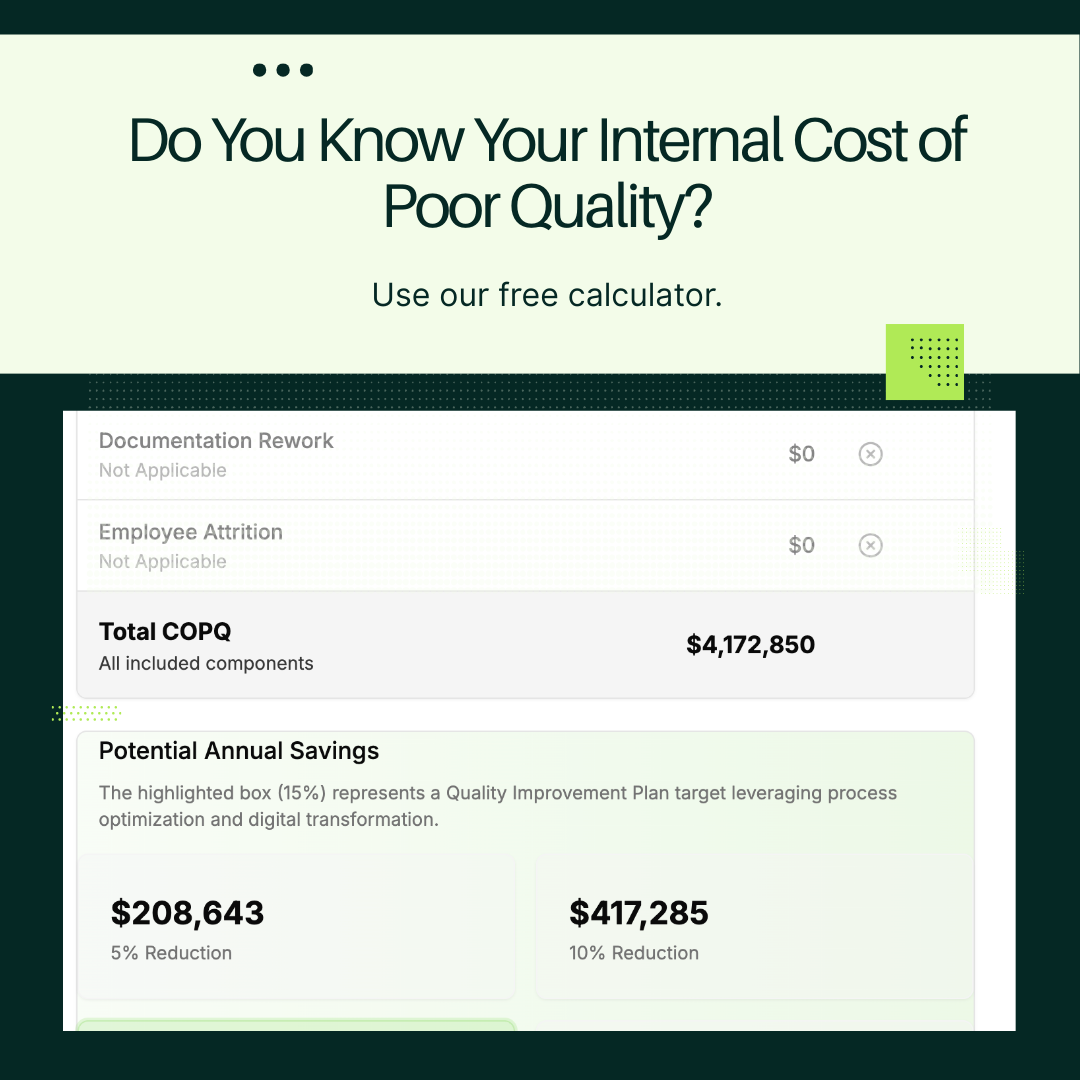
Calculate Your Cost of Poor Quality
Understand the financial impact of operational failures and how GMPKit can help you recover revenue.
Quantify Your COPQ Impact
Our COPQ calculator helps you understand the financial impact of batch failures, backlogs, and capacity constraints—providing the business case for operational recovery.


Proven Results Across Pharmaceutical Manufacturing
We've delivered measurable results across CDMOs, biopharma sites, and advanced therapy manufacturers— restoring operational stability when it matters most.
Backlog reduction in 90 days
Batch success rate improvement
Inspection readiness achieved
Successful scale-up completion
Industries We Serve
Global Experience, Local Execution:
Our team has delivered results across North America, Europe, and Asia—bringing global best practices to your local manufacturing challenges while working alongside your site teams.
Ready to Restore Operational Stability?
When your manufacturing operations are off-track and you need rapid results, let's discuss how GMPKit can deploy our proven approach to get your strategy back on track in 90-120 days.
